« Promoting research is transforming knowledge and skills into usable and marketable products»
The broad definition of research promotion that has been given by the French National Committee for Evaluating Higher Education (CNE).
Results
- Know-how
- Skills
- Software
- Database
- Platforms
- Equipments
Fundamental mission of :
-Public service, Inserm and AP-HP
strategic plans and the Articles L123-3, L123-5 and L6142-3
-Researchers
Charter and code
Providing recognition of your scientific work
Criteria for evaluation:
-HCERES
strategic plan
-Funding
-Professional career
Generating new ways of financing research
Contributing to the socio-economic development
*Research dissemination:
CV Science
Scientific communication
papers (authorship guidance ), conferences, meetings,
Communication with the general public
science festivals, workshops, Inserm associations (Gram)
Mobility of researchers
Education and training
Partners:
-Implementation officer at DR Paris 5
-Inserm Transfert
-Inserm Transfert Initiatives
-Office of Technology Transfer, Licensing & Industrial Ventures of AP-HP (OTT-LIV)
The INSERM provides a online mini-CV publishing service for researchers, on the Internet at the URL address: https://cvscience.aviesan.fr
Mission:
*Making the scientific expertise of researchers in life sciences and health more visible.
This service should enable quickly contact and easy access to relevant information about the scientific expertise of researchers.
*Fulfilling the requests for expertise from the European Union, the International community and the INSERM.
A scientific publication is an intellectual paternity acknowledgment of published results and implies a significant input for designing or conducting research.
Recommended procedures outlined by international organizations and by Aviesan :
Definition of authorship
Authorship credit should be based on :
• substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data;
• drafting the article or revising it critically for important intellectual content;
• final approval of the version to be published.
Acquisition of funding, collection of data, or general supervision of the research group, alone, does not constitute authorship.
Providing general administrative or financial support, routine technical help, writing assistance, data collection, equipment donations, technical advice, gift of reagents or samples should be named in the Acknowledgments, and the specific contribution of all authors should be described. All authors who are listed in this way should be aware of it, as some journals will require signatures of those acknowledged.
Order of authors
There are no clear rules for determining the order in which authors should be listed. The most frequently followed convention is to list authors according to their relative contributions :
- The first named author has therefore generally made the greatest contribution to the research. The student or postdoc who actually did the work goes first. The last author is usually the principal investigator or a senior scientist who oversees the experiment and guarantees the authenticity of the work.
-Co-first authoring can be helpful for the career progression of students and junior scholars when research collaborations are done. « Co-last authorship » is emerging.
-When a large multi-author group has conducted the work, the group should decide who are the authors that meet all criteria for authorship and all members of the group should be named in the Acknowledgments.
References :
Uniform Requirements for Manuscripts Submitted to Biomedical Journals: Writing and Editing for Biomedical Publication (http://www.icmje.org/)
World Association of Medical Editors (http://www.wame.org/)
European Association of Science Editors, EASE (http://www.ease.org.uk/)
Committee on Publication Ethics, COPE (http://publicationethics.org/)
Council of Science Editors, CSE (http://www.councilscienceeditors.org/)
Patients’ Association Liaison Group (GRAM) is made up of patients’ association representatives, scientists and operational managers at Inserm.
GRAM holds discussions and makes proposals regarding strategic policy and the measures to take to develop the partnership and dialogue policy between Inserm and patients’ associations. http://english.inserm.fr/about-inserm/organization-chart/committees/gram
IMPLEMENTATION MANAGER AT DR PARIS 5
http://www.idf.inserm.fr/rubriques/l-inserm-en-ile-de-france/annexes-valorisation/dr-paris-5-valorisation
•Assists and advises researchers
•Establishes research collaboration agreements involving industry partners:
-Sets up and negociates research collaboration agreements
-In charge of relations with industry partners
•Responsible for relations with Inserm-Transfert
•Contact information:
Hélène Louvel
Tel: +33 1 40 78 49 89
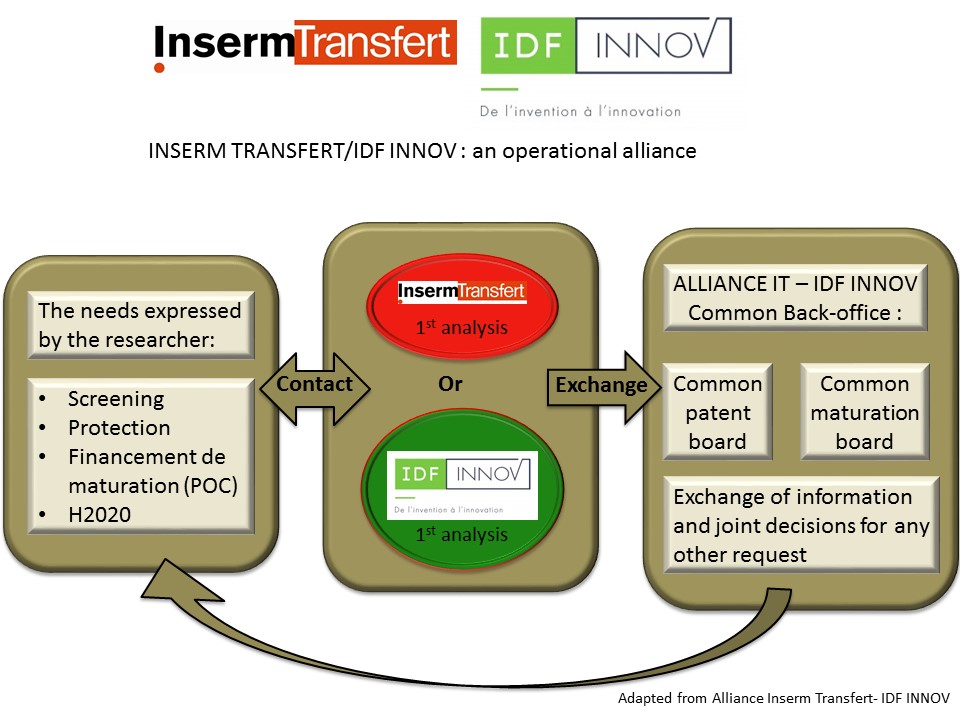
Alliance Inserm Transfert – SATT IdF Inno
INSERM TRANSFERT http://www.inserm-transfert.fr/
SATT IdF Inno http://www.idfinnov.com/en/
• Maturation and proof of concept of projects with highest industrial potential
• Intellectual property
• Industrial partnerships
• Collaborative projects, consortium and alliance management
-European research projects
-Industrial alliance management
-Public health
• Entrepreneurship
• Contact information:
-Inserm Transfert
lara.moumne@inserm-transfert.fr
-SATT IDF INNOV (within Paris Descartes University)
florence.gombert@parisdescartes.fr or fgt@idfinnov.com
INSERM TRANSFERT INITIATIVE
http://www.inserm-transfert-initiative.com/fr
• Invests in innovative early-stage life science companies and notably spin-offs from top tier French academic research centers.
• Provides financing to French start-up companies in the fields of biotechnology/biopharmaceuticals, medical devices and diagnostics.
• Contact information:
contact@it-initiative.fr
Tel: +33 1 55 03 01 00
OFFICE OF TECHNOLOGY TRANSFER, LICENSING & INDUSTRIAL VENTURES OF AP-HP (OTT-LIV)
http://ottpi.aphp.fr/
• Identifying and protecting innovations generated by AP-HP personnel.
• Technology transfer.
• Incentives for industrial partnerships on hospital sites.
• Promoting company creation based on innovations emerging from AP-HP.
• Research collaboration agreements.
• Raising awareness of and training for the challenges of protecting innovation.
• Contact information:
http://ottpi.aphp.fr/nous-contacter-2/nous-contacter/
Watch videos « Valoriser, quelle idée » (in french) / http://www.inserm-transfert.fr/fr/videos.html
Download brochures (in french)