Team leader : Philip Gorwood
Team member : Thierry Bienvenu | Jingxian Cao | Caroline Dubertret | Philibert Duriez | Yann Le Strat | Nicolas Lebrun | Cedric Lemogne | Frédéric Limosin | Jasmina Mallet | Francesca Mariuz | Julia Mattioni | Tara Petzke | Nicolas Ramoz | Sarah Tebeka | Chloe Tezenas du Moncel | Florence Thibaut | Virginie Tolle | Camille Verebi | Odile Viltart
 |
The laboratory focuses its work on finding predictive factors and / or vulnerability to addictive behaviors, including eating disorders such as anorexia nervosa, and substance misuse including alcohol dependence.
The approaches used in our laboratory are triple, phenotypic/endophenotype, developmental and genetic/epigenetic analysis of addictive behaviors. We corroborate these approaches to functional analyzes of molecular and genomic biology on in vitro models of cell cultures and in vivo in animals.
The aim of our works is to identify biomarkers of diagnosis and prognosis of addictions and in particular anorexia nervosa in order to propose the most adapted care to patients and to develop novel treatments. |
The laboratory focuses its work on finding predictive factors and / or vulnerability to addictive behaviors, including eating disorders such as anorexia nervosa, and substance misuse including alcohol dependence.
The approaches used in our laboratory are triple, phenotypic/endophenotype, developmental and genetic/epigenetic analysis of addictive behaviors. We corroborate these approaches to functional analyzes of molecular and genomic biology on in vitro models of cell cultures and in vivo in animals.
The aim of our works is to identify biomarkers of diagnosis and prognosis of addictions and in particular anorexia nervosa in order to propose the most adapted care to patients and to develop novel treatments.
Figure: overview of our research works
Addictive disorders are a broad concept that shares common characteristics. Taking into account the definition of Aviel Goodman in 1990, addictive behavior is either a source of pleasure that is sought (positive reinforcement) or a source of relief that one wants to avoid (negative reinforcement). There is a loss of control of consumption or behavior (as shown by the failure of attempts to reduce or stop) despite the existence of negative consequences. This definition is widely used in all international diagnostic criteria (DSM-5 and ICD-10), and has the particularity of being potentially compatible with dependency types.
The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, commonly referred to as the DSM-V or DSM 5, is the latest version of the American Psychiatric Association’s gold standard text on the names, symptoms, and diagnostic features of every recognized mental illness, including addictions. The DSM 5 criteria for substance use disorders are based on decades of research and clinical knowledge. This edition was published in May 2013, nearly 20 years after the original publication of the previous edition, the DSM-IV, in 1994.
Addictive disorders may be related to abuse or dependence, and may include substances (such as alcohol, cocaine, cannabis, tobacco, etc.) or behavior (such as pathological gambling, eating disorders, certain sports activities ....). Moreover, they are based on two important common mechanisms of dependence: the reward effect (increased) and the inhibitory control (decreased). Alcohol dependence is very studied in our laboratory, in fact Europe is the place in the world where the consumption of alcohol is the highest, and France is one of the five European countries where the level is more despite a recent decline.
The activation of the brain’s reward system is central to problems arising from substance use –- the rewarding feeling that people experience as a result of taking substances may be so profound that they neglect other normal activities in favor of taking the substance.
While the pharmacological mechanisms for each class of substance is different, the activation of the reward system is similar across substances in producing feelings of pleasure or euphoria, which is often referred to as a “high.”
Eating disorders (EDs) are complex and multifactorial psychiatric illnesses characterized by severe disturbances in feeding behavior leading to pathological relationships with food in both quantitative and / or qualitative terms. In DSM 5, the main disorders described are anorexia nervosa (AN), bulimia (BN) and binge eating disorder (BED).
Anorexia nervosa (AN) is defined by the persistent restriction of energy intake relative to requirements leading to significantly low body weight, an intense fear of gaining weight or becoming fat. AN has the highest mortality rate of all psychiatric disorders (10% of mortality) and is associated with severe and frequent comorbid psychiatric and somatic complications, including the highest risk for suicide attempt of 23 times. AN is therefore a major public health problem.
AN remains poorly understood and one of the main challenges is the identification of factors involved in the pattern of chronicity, relapse and severity. AN has a high genetic component with an heritability about 0.7. According to us, AN should be evaluated not only through the verbal expression of symptoms, but also through more reliable endophenotypes. The approach based on endophenotypes allow a better understanding of the physiopathology of this complex disorder and facilitate the identification of the genes involved. Thus, endophenotypes may be more suitable for detecting risk genes, because they are genetically less complex than phenotypes.
Recent studies support the idea that AN could be the consequence of aberrant reward processing and, combined with exaggerated control, could involve neural circuits implicated in reward processing and compulsivity. Altered patterns of brain activity associated with emotional and reward processing tasks, related to more or less specific stimuli of the illness, have provided important information about the mechanisms underlying AN symptoms. It has been suggested that thinness could have a reward value in AN.
| Figure: paradigm estimation of the weight of the different shapes | Figure: paradigm of satisfaction with different shapes |
 |
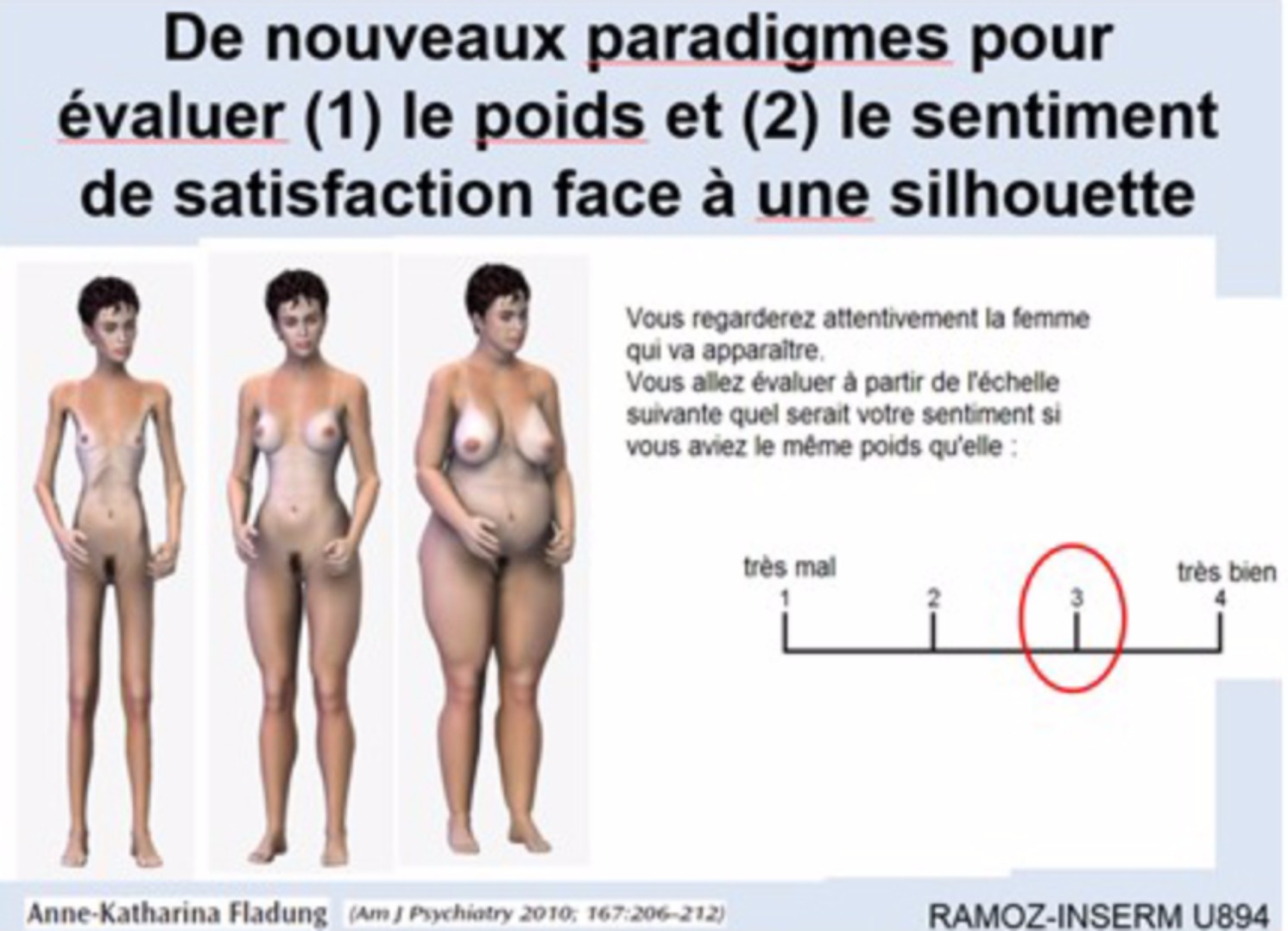 |
We have recently assessed the characteristics of the cognitive, emotional and physiologic response towards disease-specific pictures of female body shapes, in adult AN patients compared with healthy controls (HC) women. Frequency and amplitude of skin conductance response (SCR) in 71 patients with AN and 20 HC were registered during processing of stimuli of three weight- categories (over-, under- and normal weight). We then assessed the role of the Val66Met BDNF polymorphism as a potential intermediate factor. AN patients reported more positive feelings during processing of underweight stimuli and more negative feelings for normal and overweight stimuli. The SCR showed a significant group effect, AN patients showing overall higher frequency of the response. SCR within patients was more frequent during processing of underweight stimuli compared to normal and overweight stimuli. The Met allele of the BDNF gene was associated to an increased frequency of SCR in response to cues for starvation. A higher positive value of starvation, rather than more negative one of overweight, might more accurately define females with anorexia nervosa. Thus, anorexia would rather be a pleasure to thinness rather than a fear of getting fat.
Information presse INSERM 2016 06 07
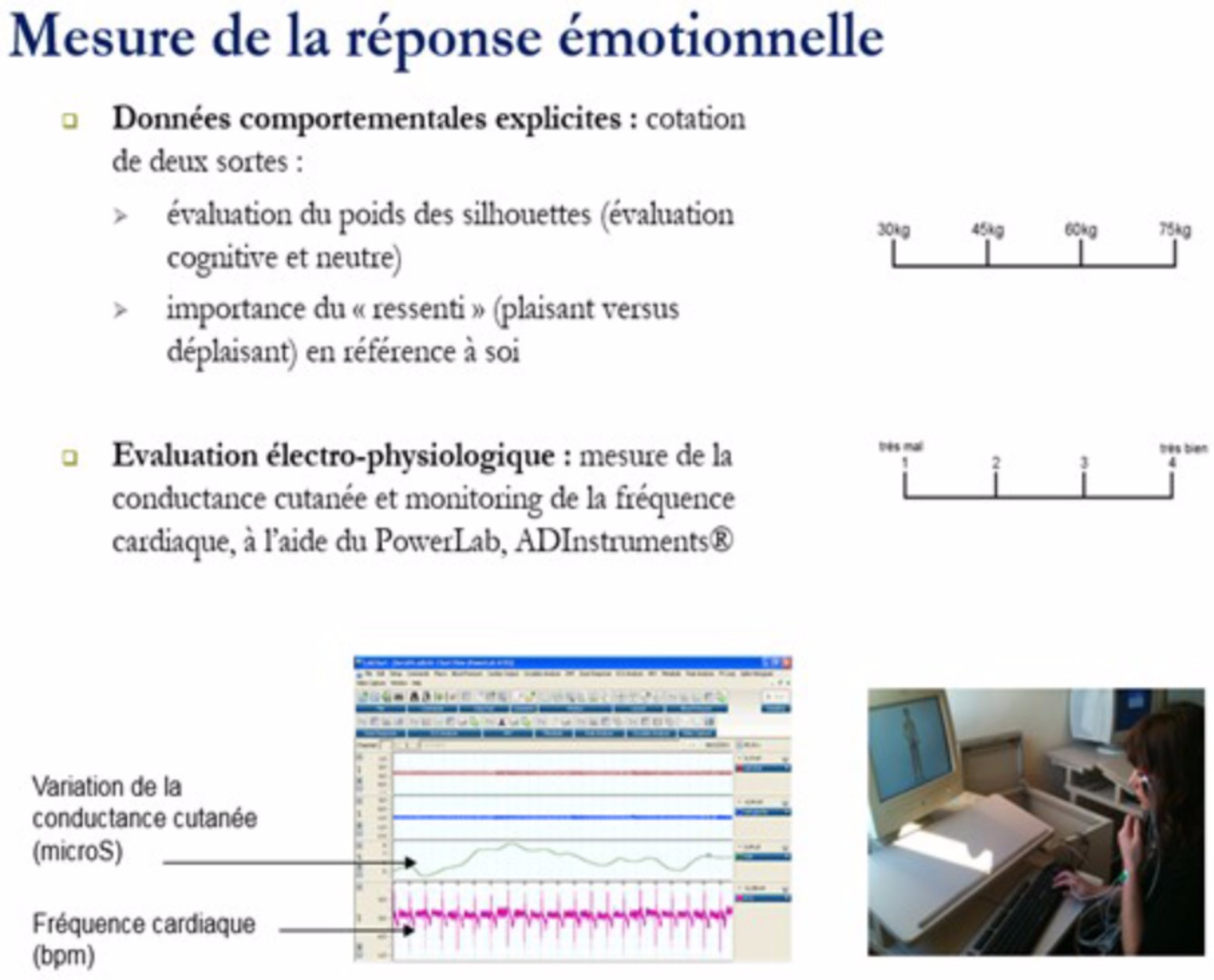
Our endophenotype approach is embraced in our approach is "phenotypic", ie it is based on what is observed. We thus seek the simple and common clinical determinants of the various addictions, making it possible to simplify the definition of the concept on which genetic studies are based (Professor Philip Gorwood's CMME Service, Sainte-Anne Hospital, Professor Caroline Dubertret's Service, Hôpital Louis Mourier, Professor Frédéric Limosin, Corentin-Celton Hospital, Cédric Lemogne Hospital, HEGP Hospital, Pr Florence Thibaut, Tarnier-Cochin Hospital, Dr. Julia Clarke and Sara Bahadori Robert Debré Hospital). In the clinical registry, studies take into account the age of onset, psychiatric comorbidity, response to therapy (psychopharmacogenetics), cognitive functioning modalities evaluated using experimental paradigms measuring impulsivity and research of immediate gratification.
The aim is to study young subjects (children, adolescents and young adults) and to allow longitudinal follow-up of these subjects or adult patients over several years.
Thus, in the past, we have focused on hyperactivity with attention deficit, which has the peculiarity of reaching subjects from childhood (before any consumption) and being a considerable risk factor for addictions The results of the study are summarized in the following articles: [Asch M, Cortese S, Perez Diaz F, Pelissolo A, Aubron V, Orejarena S, Acquaviva E, Mouren MC, Michel G, Gorwood P, Purper-Ouakil D. Psychometric properties of a junior temperament and character Inventory. Eur Child Adolesc Psychiatry. 2009, 18: 144-53], [Saudino KJ, Carter AS, Purper-Ouakil D, Gorwood P. The etiology of behavioral problems and competencies in very young twins. J Abnorm Psychol. 2008, 117: 48-62.], [Purmer-Ouakil D, Cortese S, Wohl M, Asch M, Acquaviva E, Falissard B, Michel G, Gorwood P, Mouren MC. Predictors of diagnosis delay in a clinical sample of children with attention-deficit / hyperactivity disorder. Eur Child Adolesc Psychiatry. 2007, 16: 505-9].
More recently, we have studied the effect of first cannabis use and the developmental consequences of drug dependence in young adults [Strat Y, Ramoz N, Horwood J, Falissard B, Hassler C , Romero L, Choquet M, Fergusson D, Gorwood P. First positive reactions to cannabis constitute a priority risk factor for cannabis dependence. Addiction. 2009 Oct; 104 (10): 1710-7.].
Schizophrenia and bipolar disorder are also pathologies that we study in terms of the addictions that are associated, especially for the consumption of cannabis (Pr Caroline Dubertret and Dr Jasmina Mallet, participation in the network FondaMendal Head Pr Marion Leboyer).
The laboratory looks for biological markers in anorexia nervosa [Ramoz N, Versini A, Gorwood P. Eating disorders: an overview of treatment responses and the potential impact of vulnerability genes and endophenotypes. Expert Opin Pharmacother. 2007, 8: 2029-44], possibly associated with an increased risk of suicide [Foulon C, Guelfi JD, Kipman A, Ades J, Romo L, Houdeyer K, Marquez S, Mouren MC, Rouillon F, Gorwood P. Switching To the bingeing / purging subtype of anorexia nervosa is often associated with suicidal attempts. Eur Psychiatry. 2007, 22: 513-9].
We carry out genetic and epigenetic analyzes (level of DNA methylation and expression of small non-coding RNAs, the miRNAs) in anorexia nervosa from families (parents and affecte children) or case studies versus controls.
We are participating in the International Consortium on Anorexia nervosa, GCAN [Hinney A, GCAN, The Wellcome Trust Case Control Consortium 3 (218 collaborateurs). Evidence for three genetic loci involved in both anorexia nervosa risk and variation of body mass index. Mol Psychiatry. 2016, in press; Kesselmeier, GCAN, The Wellcome Trust Case Control Consortium 3 (218 collaborateurs). High-throughput DNA methylation analysis in anorexia nervosa confirms TNXB hypermethylation. World J Biol Psychiatry. 2016, 1:1-13. Huckins LM, GCAN, The Wellcome Trust Case Control Consortium 3 (218 collaborateurs). Using ancestry-informative markers to identify fine structure across 15 populations of European origin. Eur J Hum Genet. 2014, 22(10):1190-200; Boraska V, GCAN, The Wellcome Trust Case Control Consortium 3 (218 collaborateurs). A genome-wide association study of anorexia nervosa. Mol Psychiatry. 2014, 1 9(10):1085-94]
| Figure: Automate 7900HT Fast Real-Time PCR system to genotype DNA or quantify RNA | Figure: Genotyping of a genetic variant in 384 subjects |
 |
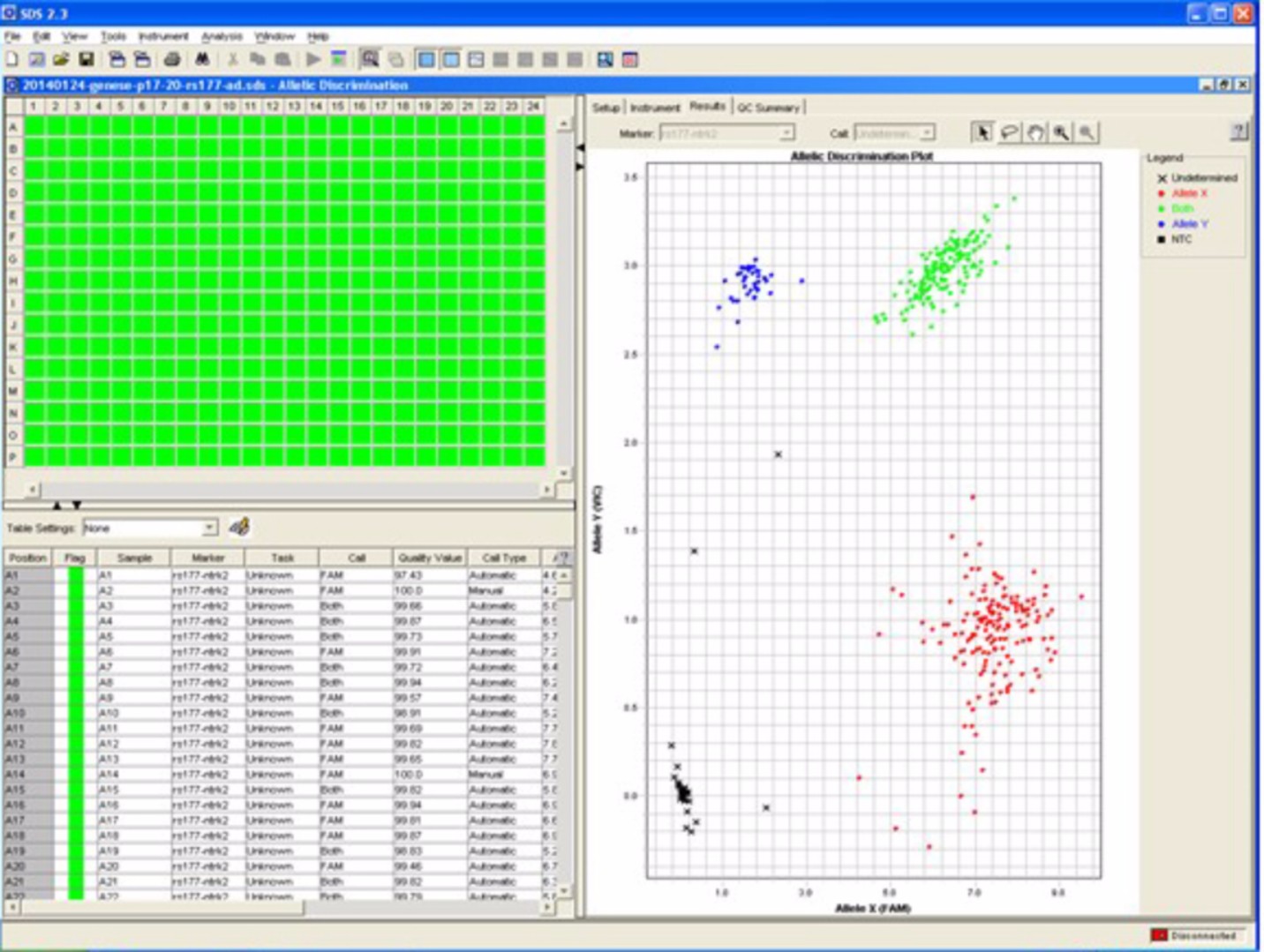 |
We are participating in the International Consortium on Anorexia nervosa, GCAN [Hinney A, GCAN, The Wellcome Trust Case Control Consortium 3 (218 collaborateurs). Evidence for three genetic loci involved in both anorexia nervosa risk and variation of body mass index. Mol Psychiatry. 2016, in press; Kesselmeier, GCAN, The Wellcome Trust Case Control Consortium 3 (218 collaborateurs). High-throughput DNA methylation analysis in anorexia nervosa confirms TNXB hypermethylation. World J Biol Psychiatry. 2016, 1:1-13. Huckins LM, GCAN, The Wellcome Trust Case Control Consortium 3 (218 collaborateurs). Using ancestry-informative markers to identify fine structure across 15 populations of European origin. Eur J Hum Genet. 2014, 22(10):1190-200; Boraska V, GCAN, The Wellcome Trust Case Control Consortium 3 (218 collaborateurs). A genome-wide association study of anorexia nervosa. Mol Psychiatry. 2014, 1 9(10):1085-94]
Figure: Statistical differences in methylation levels for 450000 DNA sites between different populations (AN: AM patients, Control: women controls, REM in remission)
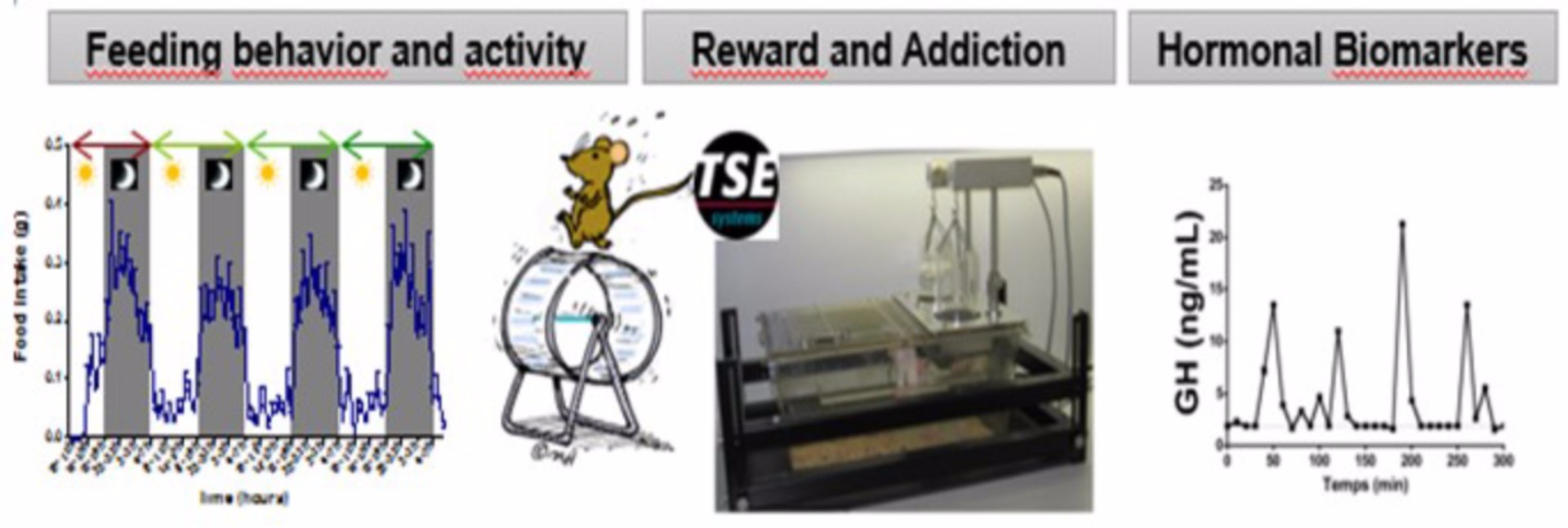
Animal models (Dr Virginie Tolle and Odile Viltart) allow us to perform in vivo functional analyzes, mainly on eating disorders. Animals carrying genetic modifications are constituted, such as mice whose gene encoding preproghrelin is invalidated. Environmental models are also produced. The combination of the two makes it possible to study the pathophysiology of eating disorders. We can study the neuroanatomic networks connecting the feeding and reward/dependency circuits. We work on the feeding behavior, reward, activity and anxiety in relation to variations in key biomarkers related to dietary behaviors.
Figure: Neuroanatomic networks linking feeding and rewar /dependency and involvement of ghrelin
Figure: Different analyzes using animal models
In summary, the general objectives that our INSERM research team pursues are (1) to analyze the specificities of the pathologies of the phenotypic spectrum of addictive behaviors that are disorders of food behavior (temperamental analysis, addictive comorbidity and genetic vulnerability).
We also (2) increase the cohort of alcohol-dependent or at-risk subjects in a cohort of 800 alcohol-dependent subjects (PHRC) followed at 2 years (face-to-face) and 10 years for vital status. Another collaboration between INSERM and the National Institute of Alcohol Abuse and Alcoholism (NIAAA) gave us the unique opportunity to have 150 families from the American Consortium Collaborative Work on the Genetics of Alcohol Dependence (COGA) By recruiting, through a collaboration with Dr Choquet (INSERM U669), the Regional Health Observatory Champagne-Ardenne, supporters of the Foundation for Research on Alcohol (FRA / Fondation de France) and MILTdeCA, A cohort of 3000 young subjects of the general population, evaluating their level of consumption of the various addictive substances and their initial level of alcohol tolerance, by comparing these data with the genetic data obtained and by a prospective study to evaluate The evolution of abuse behaviors to those of addictions.
Finally, (3) we wish to continue on in vitro models of cell culture or in vivo on animals. We are also continuing the genetic analyzes of the candidate genes to better perceive on what phenotypic dimension this gene could have an impact and this in various addictive and psychiatric disorders, especially since this gene has recently been implicated in nicotine and alcohol dependence.
Our laboratory is thus at the interface between the phenotypic characterization of the addictions and the pathologies which make it the bed and the research of the factors of genetic/epigenetic vulnerability. Our research makes it possible to concretize and enrich already important collaborations, and facilitates joint research around the complex relationship between the definition of the addiction phenotype (clinical, therapeutic, epidemiological, temperamental, neurocognitive and physiological) and potentially involved genotypes or epigenetics.
The expected benefits are therefore
- a better understanding of the mechanisms involved in the process of dysconsumption and dependence,
- the definition of subgroups of subjects of simpler determinism facilitating their recognition and thus their management,
- the discovery of vulnerability genes enabling early identification of risky subjects, adapting treatment more appropriately (pharmacogenetics), or even discovering new proteins with a significant role, thus providing new therapeutic pathways.
In conclusion, our research focuses on a multifactorial analysis of addictive behaviors based on four essential and interrelated aspects, phenotypic analysis, developmental aspects, research on genetic and epigenetic factors of vulnerability and protection, and finally their functional consequences.