Team leader : Thierry Galli
Team member : Béatrice Cholley | Lydia Danglot | Paul Heo | Ivan Kortza | Quentin Lemercier | Paul Nazac | Sébastien Nola | Paula Palacios Sanchez | David Tareste | Christian Vannier | Somya Vats | Anais Vlieghe | Shengyan Xu
 |
Our team studies the dynamics of intracellular membranes in neurons in both normal and pathological contexts. Abnormalities in endosomal or mitochondrial transport are linked to a variety of psychiatric illnesses, including depression and schizophrenia, but also neurodegenerative diseases such as Charcot-Marie-Tooth (CMT), Alzheimer's, Parkinson's and Huntington's disease. This suggests a role for membrane dynamics in the formation and maintenance of the complex morphology of neurons and their contacts. The molecular and cellular principles governing the establishment and maintenance of neuronal morphology and synaptic contacts remain poorly understood. The team's aim is to understand the mechanisms and regulation of membrane trafficking in the context of the developing brain, brain tumors, psychiatric and degenerative diseases (Parkinson's, Alzheimer's). To achieve this, we use cell and molecular biology techniques, and more specifically microscopic, proteomic and biophysical approaches to study membrane dynamics in vitro and in vivo |

The core principle of SNARE-mediated fusion
Adrien V, Bosc N, Fumat H, Tessier C, Ferreri F, Mouchabac S, Tareste D, Nuss P. Higher stress response and altered quality of life in schizophrenia patients with low membrane levels of docosahexaenoic acid. Front Psychiatry. 2023 Feb 3;14:1089724. PMID: 36816405. DOI: 10.3389/fpsyt.2023.1089724
Filippini F, Nola S, Zahraoui A, Roger K, Esmaili M, Sun J, Wojnacki J, Vlieghe A, Bun P, Blanchon S, Rain JC, Taymans JM, Chartier-Harlin MC, Guerrera C, Galli T. Secretion of VGF relies on the interplay between LRRK2 and post-Golgi v-SNAREs. Cell Rep. 2023 Mar 28;42(3):112221. PMID: 36905628. DOI: 10.1016/j.celrep.2023.112221. Download from HAL UPC
Heo P, Culver JA, Miao J, Pincet F, Mariappan M. The Get1/2 insertase forms a channel to mediate the insertion of tail-anchored proteins into the ER. Cell Rep. 2023 Jan 31;42(1):111921. PMID: 36640319. DOI: 10.1016/j.celrep.2022.111921. Download from HAL-INSERM
Niort K, Dancourt J, Boedec E, Al Amir Dache Z, Lavieu G, Tareste D. Cholesterol and Ceramide Facilitate Membrane Fusion Mediated by the Fusion Peptide of the SARS-CoV-2 Spike Protein. ACS Omega. 2023 Sep 12;8(36):32729–32739. PMID: 37720777. DOI: 10.1021/acsomega.3c03610
Vlieghe A, Niort K, Fumat H, Guigner J-M, Cohen MM, Tareste D. Role of lipids and divalent cations in membrane fusion mediated by the heptad repeat domain 1 of mitofusin. Biomolecules. 2023 Sep 2;13(9). PMID: 37759741. DOI: 10.3390/biom13091341
Hewlett B, Pratap Singh N, Vannier C, Galli T. ER-PM Contact Sites – SNARING Actors in Emerging Functions. Front. Cell Dev. Biol. 2021. Feb 11;9:635518. doi: 10.3389/fcell.2021.635518. eCollection 2021. Download from HAL-INSERM Review Article
Vats S, Galli T. Introducing secretory reticulophagy/ER-phagy (SERP), a VAMP7-dependent pathway involved in neurite growth. Autophagy. 2021 Feb 8;:1-3. doi: 10.1080/15548627.2021.1883886. [Epub ahead of print] PubMed PMID: 33554711. Download from HAL-INSERM
Gallo A, Danglot L, Giordano F, Hewlett B, Binz T, Vannier C, Galli T. Role of the Sec22b-E-Syt complex in neurite growth and ramification. J Cell Sci. 2020 Sep 15;133(18):jcs247148. doi: 10.1242/jcs.247148. PMID: 32843578. Download from HAL-INSERM
Wojnacki J, Nola S, Bun P, Cholley B, Filippini F, Pressé MT, Lipecka J, Man Lam S, N'guyen J, Simon A, Ouslimani A, Shui G, Fader CM, Colombo MI, Guerrera IC, Galli T. Role of VAMP7-Dependent Secretion of Reticulon 3 in Neurite Growth. Cell Rep. 2020 Dec 22;33(12):108536. doi: 10.1016/j.celrep.2020.108536. PubMed PMID: 33357422. Download from HAL-INSERM
Collot M, Ashokkumar P, Anton H, Boutant E, Faklaris O, Galli T, Mély Y, Danglot L, Klymchenko AS. MemBright: A Family of Fluorescent Membrane Probes for Advanced Cellular Imaging and Neuroscience. Cell Chem Biol. 2019 Apr 18;26(4):600-614.e7. doi: 10.1016/j.chembiol.2019.01.009. Epub 2019 Feb 7. PubMed PMID: 30745238
General topic
Topic 1: Role of the secretory pathway in neuronal development and plasticity
Topic 2: Role of membrane contacts in neuronal development
Bibliography
Alumni
Our group studies the dynamics of intracellular membranes in developing and mature neurons, in healthy and pathogenic contexts. It is particularly intriguing that hereditary spastic paraplegia, a family of axonal degeneration diseases, is determined by mutations in genes predominantly involved in organelle dynamics, particularly mitochondria, endoplasmic reticulum (ER), endosomes and autophagosomes. Dysfunction of mitochondrial dynamics has also been linked to a variety of neurodegenerative disorders, including Charcot-Marie-Tooth (CMT), Alzheimer, Parkinson and Huntington diseases. This certainly suggests a crucial role of membrane dynamics in the formation and maintenance of neuronal elaborated shapes. Despite a large amount of literature, the molecular and cellular principles that govern the establishment of neuronal shapes still remain to be elucidated. As coined by Karl Pfenninger, plasma membrane (PM) expansion itself is neuron's Herculean task (1). Massive membrane expansion during development and maintenance of large cell surface area during life require continuous addition of new membrane. This has been thought to occur through the fusion of secretory vesicles with the PM and several routes have been involved, transporting different components, depending on the signalling context (2,3). In addition, our recent work has shown that close contacts between the ER, where most lipids are synthesized, and the PM also play a role in neurite growth through non-vesicular lipid transfer (4). Thus, which types of secretory vesicles are involved and how non-vesicular mechanisms participate to membrane expansion is not clear at all. How the neuronal membranes are maintained during life and aging and how the underlying mechanisms may be impaired in neurodegeneration are still open questions.
Group photo of the team
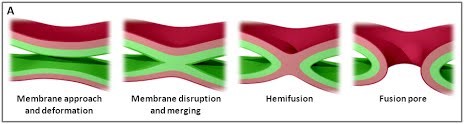
We are using a multidisciplinary and multiscale approach ranging from molecules to cells and animals, focusing on the proteins which are at the heart of intracellular membrane fusion events. SNARE proteins constitute the main molecular machinery for docking and fusion of intracellular membranes. SNAREs are transmembrane proteins that are classified into R-SNAREs, mainly corresponding to vesicular (v)-SNAREs and Q-SNAREs, mainly target (t)-SNAREs. A functional fusogenic SNARE complex is composed of three Q-SNAREs (generally on the acceptor compartment) and one R-SNARE (on the opposing membrane, often a vesicle). The assembly of R/Q-SNAREs between two membranes in the form of a parallel coiled-coil of four heptad repeat (HR) domains generates a trans-SNARE complex, which docks the lipid bilayers and brings them into close proximity to promote fusion. This process often includes the passage through a hemifusion intermediate state, where the outer monolayers have fused while the inner monolayers remain separated. SNARE-mediated membrane docking, by shortening the inter-bilayer distance, may also favour lipid transfer before or independently of fusion.
 |
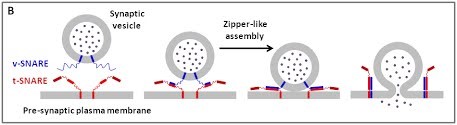 |
Figure 1. (A) The various stages of membrane fusion; adapted from (Jahn et al., Curr Opin Cell Biol 2002).
(B) The core principle of SNARE-mediated membrane fusion.
Neurite growth during neuronal development has more generally been thought to be mediated by SNARE-dependent fusion of vesicles with the PM. In mammals, the brevin v-SNAREs VAMP2, VAMP4 and the Longin v-SNARE VAMP7 have been implicated in neuronal growth and their involvement seems to depend on signalling pathways such as those involving integrin, Netrin, or NGF [reviewed in (2,3)]. Our team focuses particularly on the role of the brevins VAMP2 and VAMP3 and on the Longin v-SNAREs VAMP7 and Sec22b.
Figure 2. Schematic representation of neuritogenesis followed by synaptogenesis
Mitochondria form a highly dynamic network of organelles which constantly move, fuse and divide within cells. This dynamic nature of mitochondria is essential for cellular function, and mutations in mitochondrial fusion and fission proteins lead to important human diseases. Mitofusins, Mfn1 and Mfn2, are large transmembrane GTPase proteins of the outer mitochondrial membrane that are crucial for mitochondrial fusion. Mitofusins possess, in addition to their GTPase and transmembrane domains, two heptad repeat domains, HR1 and HR2. Mutations in any of these functional domains impair Mitofusin function, and can lead to CMT2A disease in the case of mutations in Mfn2, but their exact role in mitochondrial fusion remains unknown (for more details: https://sites.google.com/site/insermu950/Biophysics-of-membrane-fusion ). The X-ray crystal structure of the HR2 domain of Mfn1 revealed an antiparallel coiled-coil dimer suggesting a role for HR2 in mitochondrial docking. Mitochondrial fusion is also regulated by specific lipids such as cardiolipin (CL), phosphatidylethanolamine (PE) and phosphatidic acid (PA). These lipids are believed to be exchanged between ER and mitochondria via membrane contact sites (MCS). Interestingly, ER and mitochondria were shown to be physically connected by homotypic and heterotypic complexes involving Mfn2 on the ER and Mfn1 or Mfn2 on the outer mitochondrial membrane. Our main goals are therefore to elucidate the molecular mechanisms by which Mitofusins mediate mitochondrial fusion and how this process is regulated by lipids, and functionally coupled to the lipid transfer machinery at ER-mitochondria MCS.
 Figure 3. Snapshot of mitochondria undergoing fusion and fission
Figure 3. Snapshot of mitochondria undergoing fusion and fission
|
|
Main papers: Zylbersztejn et al. J Cell Biol 2012; Danglot, Freret et al, J Neurosci, 2012 ; Danglot, Zylbersztejn et al, J Neurosci, 2012 ; Burgo et al, Dev Cell, 2012, Burgo et al., J Biol Chem 2013 ; Molino et al., Cell Logist 2015; Kuster et al, J Biol Chem 2015
|
We further found that VAMP7 mediates exocytosis of Golgi-derived vesicles (10), and that its deletion alters GPI-anchored proteins’ exocytosis and sphingolipid membrane composition (11). VAMP7 exocytosis is regulated by IGF-1 and phosphorylation of a tyrosine in the amino-terminal Longin domain (12).
In addition, following up on previous studies, we further deciphered the interactome of VAMP7 and characterized the function of its partners: Varp, GolginA4, Rab21, MACF-1, Kif5a (10), SNAP-47 and SNAP-29 (13) in exocytosis and neurite growth.
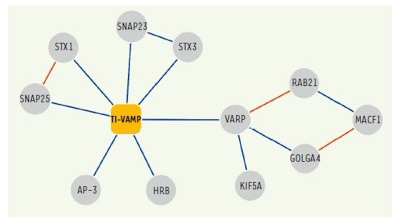 Figure 4. Network of molecular interactions of TI-VAMP/VAMP-7.
Figure 4. Network of molecular interactions of TI-VAMP/VAMP-7.
Interactions identified in our two-hybrid screens and / or biochemical approaches are shown in blue.
(Burgo et al., 2012a)
The role of VAMP7 in secretory mechanisms in immune (B and T lymphocytes and dendritic cells) and blood cells has been the topic of several collaborations (14–17). The VAMP7 KO mouse was instrumental in the demonstration of the importance of VAMP7-mediated secretory mechanisms in the immune system.
We have achieved to characterize the molecular network of VAMP7 and to further decipher its functions in secretion, neurite growth and brain signaling. Future studies are required to understand more precisely the contribution of each secretory mechanism in vivo.
 |
Main papers: Petkovic et al, Nature Cell Biol, 2014, Gallo et al, Annu. Rev. Cell Dev. Biol, 2016 we recently discovered that the v-SNARE Sec22b, a highly conserved ER-localized SNARE that plays a fundamental role in vesicle fusion within the early secretory pathway, had an additional and unexpected function: to be involved in the docking of ER and PM membranes rather than to promote their fusion (4). ER-PM contact sites were first identified in the 1950s, and were recently shown to be important for non-vesicular transport of lipids. Neurons provide a striking example of cells with extensive cortical ER and abundant ER-PM contact sites, but little is known about the molecular composition and function of these contact sites. We further found that the non-fusogenic Sec22b-Stx1 complex plays an important role in neurite growth. |
1. Pfenninger KH. Plasma membrane expansion: a neuron’s Herculean task. Nat Rev Neurosci. 2009 Apr;10(4):251–261.
2. Wojnacki J, Galli T. Membrane traffic during axon development. Dev Neurobiol. 2016 Nov;76(11):1185–1200.
3. Daste F, Galli T, Tareste D. Structure and function of longin SNAREs. J Cell Sci. 2015 Dec 1;128(23):4263–4272.
4. Petkovic M, Jemaiel A, Daste F, Specht CG, Izeddin I, Vorkel D, et al. The SNARE Sec22b has a non-fusogenic function in plasma membrane expansion. Nat Cell Biol. 2014 May;16(5):434–444.
5. Zylbersztejn K, Petkovic M, Burgo A, Deck M, Garel S, Marcos S, et al. The vesicular SNARE Synaptobrevin is required for Semaphorin 3A axonal repulsion. J Cell Biol. 2012 Jan 9;196(1):37–46.
6. Danglot L, Freret T, Le Roux N, Narboux Nême N, Burgo A, Hyenne V, et al. Vezatin is essential for dendritic spine morphogenesis and functional synaptic maturation. J Neurosci. 2012 Jun 27;32(26):9007–9022.
7. Danglot L, Zylbersztejn K, Petkovic M, Gauberti M, Meziane H, Combe R, et al. Absence of TI-VAMP/Vamp7 leads to increased anxiety in mice. J Neurosci. 2012 Feb 8;32(6):1962–1968.
8. Sato M, Yoshimura S, Hirai R, Goto A, Kunii M, Atik N, et al. The role of VAMP7/TI-VAMP in cell polarity and lysosomal exocytosis in vivo. Traffic. 2011 Oct;12(10):1383–1393.
9. Ghosh D, Pinto S, Danglot L, Vandewauw I, Segal A, Van Ranst N, et al. VAMP7 regulates constitutive membrane incorporation of the cold-activated channel TRPM8. Nat Commun. 2016 Feb 4;7:10489.
10. Burgo A, Proux-Gillardeaux V, Sotirakis E, Bun P, Casano A, Verraes A, et al. A molecular network for the transport of the TI-VAMP/VAMP7 vesicles from cell center to periphery. Dev Cell. 2012 Jul 17;23(1):166–180.
11. Molino D, Nola S, Lam SM, Verraes A, Proux-Gillardeaux V, Boncompain G, et al. Role of tetanus neurotoxin insensitive vesicle-associated membrane protein in membrane domains transport and homeostasis. Cell Logist. 2015 Mar;5(1):e1025182.
12. Burgo A, Casano AM, Kuster A, Arold ST, Wang G, Nola S, et al. Increased activity of the vesicular soluble N-ethylmaleimide-sensitive factor attachment protein receptor TI-VAMP/VAMP7 by tyrosine phosphorylation in the Longin domain. J Biol Chem. 2013 Apr 26;288(17):11960–11972.
13. Kuster A, Nola S, Dingli F, Vacca B, Gauchy C, Beaujouan J-C, et al. The Q-soluble N-Ethylmaleimide-sensitive Factor Attachment Protein Receptor (Q-SNARE) SNAP-47 Regulates Trafficking of Selected Vesicle-associated Membrane Proteins (VAMPs). J Biol Chem. 2015 Nov 20;290(47):28056–28069.
14. Chiaruttini G, Piperno GM, Jouve M, De Nardi F, Larghi P, Peden AA, et al. The SNARE VAMP7 Regulates Exocytic Trafficking of Interleukin-12 in Dendritic Cells. Cell Rep. 2016 Mar 22;14(11):2624–2636.
15. Larghi P, Williamson DJ, Carpier J-M, Dogniaux S, Chemin K, Bohineust A, et al. VAMP7 controls T cell activation by regulating the recruitment and phosphorylation of vesicular Lat at TCR-activation sites. Nat Immunol. 2013 Jul;14(7):723–731.
16. Schena F, Volpi S, Faliti CE, Penco F, Santi S, Proietti M, et al. Dependence of immunoglobulin class switch recombination in B cells on vesicular release of ATP and CD73 ectonucleotidase activity. Cell Rep. 2013 Jun 27;3(6):1824–1831.
17. Koseoglu S, Peters CG, Fitch-Tewfik JL, Aisiku O, Danglot L, Galli T, et al. VAMP-7 links granule exocytosis to actin reorganization during platelet activation. Blood. 2015 Jul 30;126(5):651–660.