Team leader : María Cecilia Angulo
Team member : Roxanne Bancel Vega | Julie Cognet | Eduardo Fernandez | Cristobal Ibaceta | Hasni Khelfaoui | Fabrice Plaisier | Beata Turanska
|
|
The team is interested in understanding the roles of neuron-oligodendroglia interactions during postnatal cortical development and in pathological conditions. We investigate how neuronal activity regulates the function of oligodendrocyte (OL) lineage cells and, in turn, how these cells and myelin affects neuronal activity, influencing sensorimotor and cognitive functions. Our first research line is focused on the study of the close relationship of GABAergic interneurons and oligodendrocyte precursor cells (OPCs) in the developing neocortex. We propose that early interneuron-oligodendroglia interactions are crucial to establish the proper execution of complex brain processes, and that cortical impairments in neurodevelopmental and myelin-related disorders are caused, at least in part, by an abnormal interneuron myelination. The second research line seeks to evaluate the role of neuronal activity in promoting myelin regeneration in preclinical models. We study whether and which neuronal signaling mechanisms control the function of OPCs and OLs and whether stimulating neuron-oligodendroglia interactions improves remyelination. |

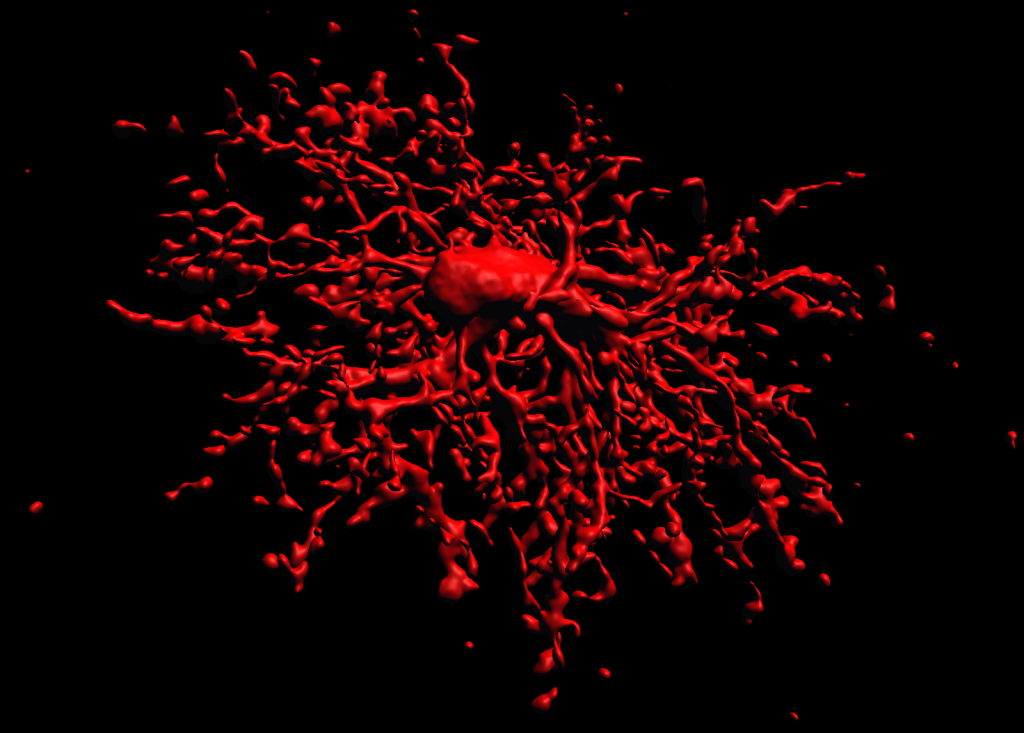
3D reconstruction of an oligodendrocyte precursor cell
Benamer N, Vidal M, Balia M, Angulo MC (2020) Myelination of parvalbumin interneurons shapes the function of cortical sensory inhibitory circuits. Nat Commun. 2020 Oct 13;11(1):5151. doi: 10.1038/s41467-020-18984-7.
Orduz D*, Benamer N*, Ortolani D*, Coppola E, Vigier L, Pierani A, Angulo MC (2019) Developmental cell death regulates lineage-related interneuron-oligodendroglia functional clusters and oligodendrocyte homeostasis. Nat Commun. 2019 Sep 18;10(1):4249. doi: 10.1038/s41467-019-11904-4. *Contributed equally. Featured article highlighted by the editor
Ortiz FC*, Habermacher C*, Graciarena M, Houry P-Y, Nishiyama A, Nait Oumesmar B, Angulo MC (2019) Neuronal activity in vivo enhances functional myelin repair. JCI Insight 4(9): e123434 PubMed PMID: 30896448; PubMed Central PMCID: PMC6538342. *Contributed equally.
Riva M*, Genescu I*, Habermacher C, Orduz D, Ledonne F, Rijli FM, López-Bendito G, Coppola E, Garel S, Angulo MC, Pierani A. Activity-dependent death of transient Cajal-Retzius neurons is required for functional cortical wiring. Elife. 2019 Dec 31;8. doi: 10.7554/eLife.50503. *Contributed equally.
Balia M, Benamer N, Angulo MC. A specific GABAergic synapse onto oligodendrocyte precursors does not regulate cortical oligodendrogenesis. Glia. 2017 Nov;65(11):1821-1832. doi: 10.1002/glia.23197.
The principal function of oligodendrocytes in the central nervous system (CNS) is to produce the myelin sheaths that insulate axons and increase the conduction velocity of action potentials. The process of myelination occurs progressively during development in humans and rodents and continues through juvenile stages. Oligodendrocytes derive from oligodendrocyte precursor cells (OPCs) expressing the proteoglycan NG2, also called NG2 cells. By their capacity to generate oligodendrocytes, OPCs play a central role in myelination during postnatal development. Furthermore, this class of progenitors also persists in the mature CNS where they continue to proliferate and generate myelinating oligodendrocytes throughout life. After a demyelinating insult, spontaneous remyelination of axons can occur because OPCs repopulate demyelinating lesions and regenerate oligodendrocytes. OPCs are therefore multifaceted cells that influence brain development and function. Since they are a main pool of progenitors in young and adult stages, they also constitute an important therapeutic target in demyelinating diseases such as Multiple Sclerosis.
1) Interactions between GABAergic interneurons and OPCs in the somatosensory cortex
Although OPCs and interneurons accomplish very different functions (respectively as a glial cell and a neuron), these cells are born from the same embryonic origin, migrate tangentially to the cortex and, once they invade cortical layers, interneurons transiently innervate OPCs through functional synapses. In continuity with our previous studies, we are studying whether these synapses regulate the maturation of OPCs onto mature oligodendrocytes, influencing the construction of neuronal circuits. In addition, we are analyzing the relationship between different subpopulations of OPCs and their ontogenetically related interneurons, and its potential implication in circuit assembly and psychiatric disorders.
2) Interactions between axons and OPCs in white matter
In Multiple Sclerosis (MS) an impaired impulse conduction arises when the immune system attacks myelin sheaths causing the loss of myelin, OL death and a subsequent neurodegeneration associated with functional and cognitive disabilities. However, despite the low regeneration capacity of the CNS, a production of newly formed OLs and a partial myelin repair can occur in this disease. This OL regeneration in response to injury depends mostly on the ability of OPCs to proliferate and differentiate in the damaged site. Several studies have demonstrated that neuronal activity impacts the fate of OPCs and modulates myelination. However, the underlying molecular and cellular mechanisms linking neuronal activity with OPC fate and myelin production are still poorly understood. In addition, while it is known that normal myelination is an activity-dependent process, it is less known whether remyelination is an activity-dependent process in pathological conditions. Our second line seeks to evaluate in vivo the regulation of activity-dependent oligodendrogenesis during myelin repair and to define the signaling and molecular mechanisms implicated in this process.
Our scientific strategy should identify new molecular and cellular mechanisms involved in OPC dynamics, oligodendrogenesis, myelination and myelin repair. It could also open a new route to understand the cellular processes that might account for modifications during behavior and disease and promote more efficient remyelination in demyelinating diseases.
3d Reconstruction of a biocytin-labeled OPC (NG2 Cell)