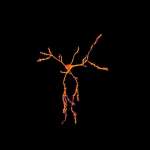
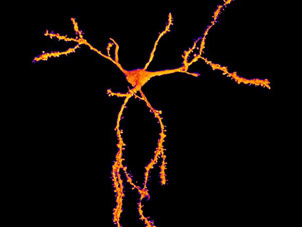
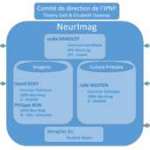
The NeurImag Imaging Facility is a core facility, located on the second floor of the Institut of Pyschiatry and Neurosciences of Paris (IPNP). The NeurImag Imaging Facility provides support for in-house and external scientists in using light microscopy techniques as well as image processing and analysis for their research projects.
Lydia Danglot (PhD, Scientific director), Sylvain Jeannin (IE), Laurianne Beynac (IE) and Philippe Bun (PhD, Staff Scientist) are currently managing the NeurImag Imaging Facility, comprising 4 advanced microscope systems and two workstations for image analysis.
Since Neuroscience is a major research topic at IPNP, the NeurImag Imaging Facility addresses imaging techniques related to issues faced in this field. Imaging techniques currently available cover a wide range of spatiotemporal resolution, ranging from video-microscopy to super-resolution imaging (PALM, STORM, STED 3D and SIM). If you wish to discuss about the potential and decisive contribution of imaging techniques to your research project, do not hesitate to contact us.
Neurimag Imaging Facility provides support for image and data analysis. Two workstations are also available if you wish to use specific software programs (FIJI, Volocity, Neurolucida, Icy ...).
Neurimag Imaging Facility is fully committed to survey and test the latest technological and methodological developments that can potentially be useful for Neuroscience. We have undertaken to streamline the use of super-resolution imaging as well as advanced photomanipulation techniques such as laser ablation and photo-uncaging, and eventually tissue clarification to in-depth imaging.
|
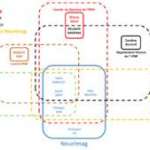
Facility map
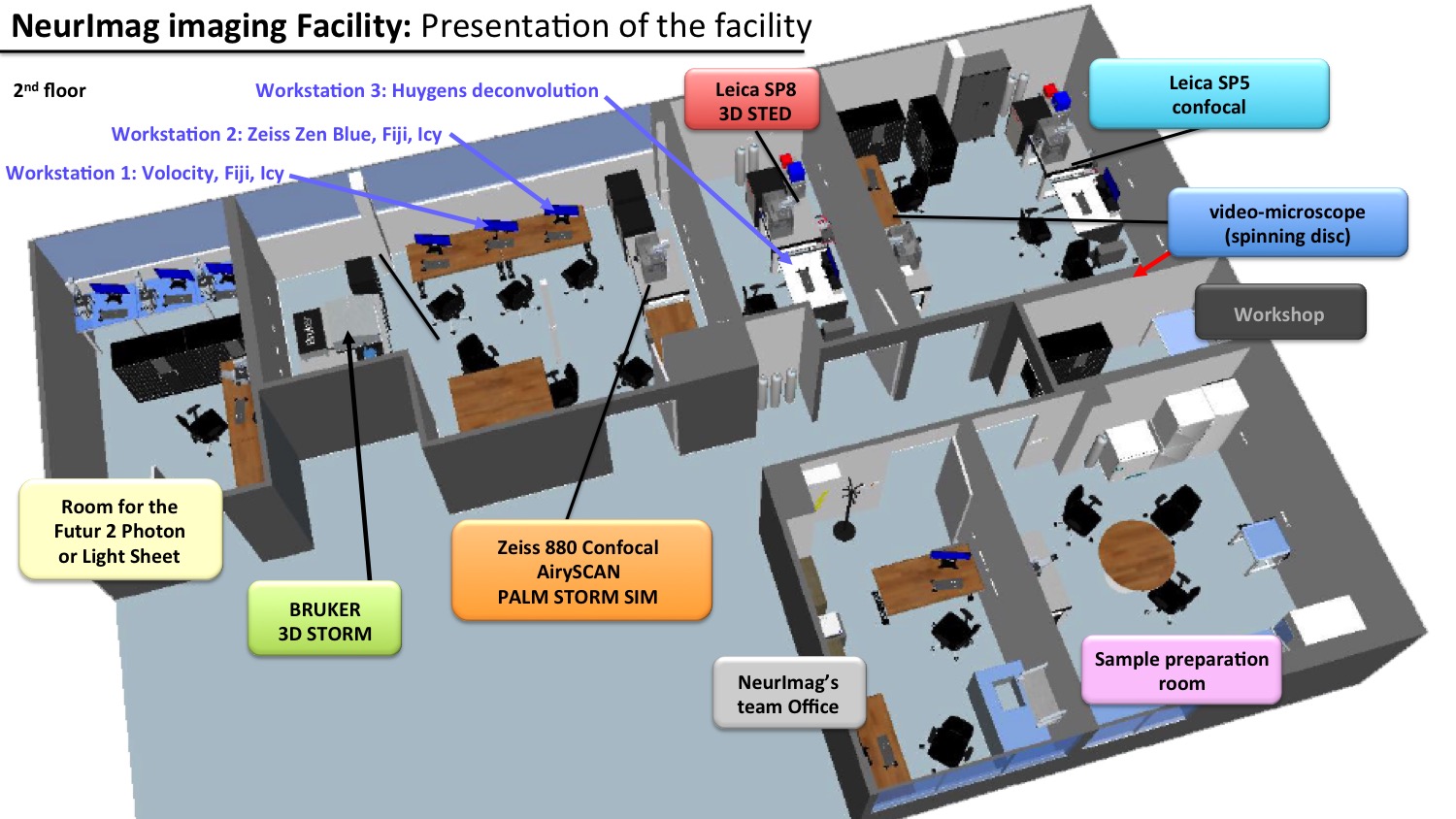
|
The schematic is here to provide help in choosing the best suited microscopy/technique available in the facility for your research project.
For more details, contact the facility staff: neurimag.ipnp@inserm.fr



NeurImag is one of the four technological core facilities at the Institute of Psychiatry and Neuroscience of Paris (IPNP). The main missions of the NeurImag Imaging Core Facility are to provide:
ASSISTANCE to all academics (IPNP and External research groups) and private companies from the process of sample preparation to microscopic imaging.
Keywords: Walk-in Advice, Microscope Selection and Training, Primary Cell Preparation, Planning of Project and Follow-up Meeting, Image Processing and Visualization, Data Transfer and Storage.
FACILITATING the usage of conventional and advanced fluorescence techniques:
- Optical sectioning:
Wide-field (no sectioning) and Confocal Scanning microscopes
Spinning-disk and Swept-field microscopes
Total Internal Reflection (TIRF)
- Monitoring Molecular and Subcellular Dynamics and Interactions:
Fluorescence Recovery After Photobleaching (FRAP)
Forster Resonance Energy Transfer (FRET)
Fluorescence Lifetime Imaging (FLIM)
Fluorescence Correlation Spectroscopy (FCS)
- Recording High Spatial and/or Temporal Resolution:
Simultaneous dual-view spinning-disk
Confocal Airyscan (and Fast Airyscan)
3D Structured Illumination Microscopy (SIM)
3D Stimulated Emission Depletion (STED)
Single-Molecule Localization (PALM*, 3D dSTORM**)
*PhotoActivated Localization microscopy, **direct Stochastic Optical Reconstruction Microscopy
- Light manipulation:
Photo-ablation, Photo-activation, Photo-uncaging
PROVIDING Image and Data Analysis support:
- Commercial and Home-written Open Source image and data analysis software.
OFFERING Biological Sample Preparation expertise and support:
- Preparation and Viability Maintenance:
Commercially available immortal cell lines
Primary cell culture (Hippocampal and Cortical Neurons, Glial and Microglial Cells)
- Development and Optimization of Imaging-related Protocols:
Transfection methods
Immunostaining
ORGANIZING courses on microscopy-related topics (Inserm, CNRS, Univ. Paris, Univ. PSL), introductory hands-on sessions to general audience, pre-evaluation of commercial equipment with technology partners
Conventional fluorescence imaging
| |
Widefield fluorescence microscopy
|
|
| |
Laser scanning confocal microscopy
|
|
| |
Spinning disc confocal system
|
|
| |
Video microscopy and slide scanner system
|
|
| |
Swept-field confocal system
|
|
Advanced fluorescence techniques
| |
Total internal reflection fluorescence microscopy (TIRF)
|
|
| |
Fluorescence Correlation Spectroscopy (FCS)
|
|
| |
Fluorescence Cross-Correlation Spectroscopy (FCCS)
|
|
| |
Fluorescence lifetime Imaging microscopy (FLIM)
|
|
| |
Fluorescence resonance energy transfer (FRET)
|
|
| |
Fluorescence recovery after photobleaching (FRAP)
|
|
| |
Laser ablation and Photon uncaging
|
|
Super resolution fluorescence techniques
| |
Structured Illumination Microscopy (SIM)
|
|
| |
Stimulated Emission Depletion microscopy (STED)
|
|
| |
Photoactivated localization microscopy (PALM)
|
|
| |
Stochastical Optical Reconstruction Microscopy (STORM)
|
|
Data and Image Analysis
| |
Image Deconvolution
|
|
| |
Workstation for data processing and analysis (open sources freeware)
|
|
| |
Data analysis custom made solution
|
|
| |
Workstations for data processing and analysis
|
|
| |
(commercial software)
|
|
Primary cells
| |
Hippocampus neurons
|
|
| |
Cortical neurons
|
|
| |
Glial cells
|
|
| |
Microglial cells
|
|
Sample preparation
| |
Coating of glass coverslip
|
|
| |
Transfection
|
|
| |
Immunostaining
|
|
Medium
| |
Conditioned medium
|
|
| |
Glial cells medium
|
|
|
Leica SP5 STED CW

|
Leica SP8 STED 3X

|
Zeiss LSM880 - Elyra PS1

|
|
Leica - Spinning disk X1

|
Zeiss Axioplan 2

|
Zeiss widefield scanner

|
|
Bruker Vutara

|
Workstations

|
Booking
|
|
Additional services

|
|
|
Leica SP5 STED CW

Inverted confocal laser scanning microscope with environment control, visible laser (Argon, 561nm and 633 nm), three photomultiplier modules (PMTs), 1 highly sensitive detector (HyD), motorized XY-stage, Z-axis piezo positioning and resonant scanner (8 kHz). STED CW module with a depletion laser line at 592 nm.
KEY FEATURES
User-friendly and intuitive software interface for immediate ease of use.
The microscope is fully enclosed and equipped with chamber heater and CO2 gas mixer (Life Imaging Services).
Detection is done with 3 PMTS, one of which for transmitted light imaging and 1 highly sensitive hybrid detector.
The microscope is equipped with a resonant scanner (8 kHz) for fast confocal imaging.
ADVANCED CAPABILITIES
In addition to an intuitive and compact software interface, making confocal imaging easy to pick up, experiments requiring FRAP (Fluorescence Recovery After Photobleaching) and ratiometric FRET (Fluorescence/Förster Resonance Transfer Energy) can be carried out on this microscope. Multi-position and Tile Scan imaging are also available.
AVAILABLE OBJECTIVES
.jpg)
LASERS
.jpg)
FILTERS FOR OBSERVATION AND ACQUISITION
Observation (oculars):
.jpg)
Acquisition:
Acousto-Optical Beam splitter (AOBS) technology with a freely selectable emission detection range. No fluorescence emission filter cubes.
DETECTION
3 photomultiplier modules (PMTs), one of which for transmitted light imaging (t-PMT)
1 highly sensitive hybrid detector (HyD)
SUPER-RESOLUTION TECHNOLOGY
STimulated Emission Depletion (STED) (with 592 nm laser lines) enables to break the diffraction limit and can reach resolutions up to approx. 50 nm (lateral) and approx. 200 nm (axial). Brielfy, this technique relies on a diffraction-limited spot that is excited at one wavelength (i.e. excitation pulse) while a super-imposed red-shifted second laser beam projected to a donut-shape (i.e. continuous STED laser), depletes all emission (or de-excitation by stimulated emission). This consequently leaves only a central focal spot where the size defines the resolution that can be tuned by the intensity of the STED laser (i.e. depletion laser). In particular, the use of continuous STED laser at 592 nm should be combined with time-gated detection to further improve the resolution. We recommend that you use the LEICA SP8 – STED 3X. Note that super-resolution STED imaging uses high laser power to induce stimulated emission that can damage samples as well as specific mounting media and preferred fluorophores should be used to enhance the efficiency of imaging.
For more information on this technique, see iBiology STED or Leica STED3X.
For sample preparation, see Leica sample preparation recommendation.
MISCELLENEAOUS
The facility provides microscopy chambers adapted for live cell imaging (Chamlide).
Best suited for: Fixed and Live samples, High-speed confocal imaging, High-resolution imaging
For more information, contact Neurimag facility.
Return
Leica SP8 STED 3X

Inverted confocal laser scanning microscope with white-light laser, gated detection on two hybrid detectors (HyDs) for super-resolution 3-colors STED imaging (592 nm and 775 nm depletion laser).
KEY FEATURES
Improved user interface for confocal imaging and optimized for fast plate reading: Navigator Module.
White light laser in combination with Argon and UV laser enables up to 14 different laser lines to be used.
Imaging can be done using gated detection on two hybrid detectors (preventing collection light reflection and/or autofluorescence)
93x glycerol-immersion objective is available, and motorized correction is implemented.
Lightning solution can alternatively be switched on for online adaptive deconvolution of confocal and super-resolution images.
ADVANCED CAPABILITIES
In addition to user-friendly confocal imaging, experiments requiring FRAP (Fluorescence Recovery After Photobleaching) and ratiometric FRET (Fluorescence/Förster Resonance Transfer Energy) can be carried out on this microscope. Multi-position and Tile Scan imaging are also available. Two deconvolution solution are available in the software (Huygens Professional or Lightning) to process confocal and STED images.
AVAILABLE OBJECTIVES
.jpg)
* motorized correction is available
** this objective lens is not mounted. If you need it contact the staff.
LASERS
.jpg)
FILTERS FOR OBSERVATION AND ACQUISITION
Observation (oculars):
.jpg)
Acquisition:
Acousto-Optical Beam splitter (AOBS) technology with a freely selectable emission detection range. No fluorescence emission filter cubes.
DETECTION
2 highly sensitive hybrid detectors (HyDs) + time-gating (photon selection by time arrival on detectors)
2 photomultiplier modules (PMTs)
SUPER-RESOLUTION TECHNOLOGY
STimulated Emission Depletion (STED) 3D (with 592 nm and pulsed 775nm laser lines) enables to break the diffraction limit and can reach resolutions up to approx. 50 nm (lateral) and approx. 200 nm (axial). Brielfy, this technique relies on a diffraction-limited spot that is excited at one wavelength (i.e. excitation pulse) while a super-imposed red-shifted second laser beam projected to a donut-shape (i.e. STED pulse for 775nm or continuous STED laser for 592nm), depletes all emission (or de-excitation by stimulated emission). This consequently leaves only a central focal spot where the size defines the resolution that can be tuned by the intensity of the STED laser (i.e. depletion laser). The use of hybrid detectors while performing super-resolution STED imaging is essential. In particular, the use of continuous STED laser at 592 nm should be combined with time-gated detection. Note that super-resolution STED imaging uses high laser power to induce stimulated emission that can damage samples as well as specific mounting media and preferred fluorophores should be used to enhance the efficiency of imaging.
For more information on this technique, see iBiology STED or Leica STED3X.
For sample preparation, see Leica sample preparation recommendation.
Best suited for: Fixed samples, Deconvolution solution, Confocal imaging, Plate reading, Super-resolution STED imaging.
For more information, contact Neurimag facility.
Return
Zeiss LSM880 - Elyra PS1

Multimodal inverted microscopy system with environment control that can be used either as a confocal laser scanning (Airyscan detection mode available) or Widefield (laser illumination in Epifluorescence, HiLo or TIRF mode) microscope coupled to two super-resolution techniques such as SIM (Structured Illumination Microscopy) and PALM/dSTORM 3D (PhotoActivated Localization Microscopy / direct STochastic Optical Reconstruction Microscopy).
KEY FEATURES
Confocal imaging with improved spatial resolution using Airyscan mode of detection as well as High-speed confocal imaging using Fast Airyscan option are available.
Microscope can perform Widefield, HiLo or TIRF imaging mode. Available emission filters are shown below.
Focus control is achieved with Definite Focus.
Microscope top stage incubator (Tokai Hit ELYX-SET) for precise temperature and CO2 control is available upon request for live cell imaging. Microscope is driven by Zen Black (version 2.3 SP1), and online processing of Airyscan images is available using Zen Blue on Workstation 2.
ADVANCED CAPABILITIES
In addition to improved (spatio-temporal) confocal and widefield (Epifluorescence, HiLo, TIRF) imaging, experiments requiring FRAP (Fluorescence Recovery After Photobleaching), ratiometric FRET (Fluorescence/Förster Resonance Transfer Energy) and Raster-scan Image Correlation Spectroscopy techniques can be carried out on this microscope. Multi-position and Tile Scan imaging are also available.
AVAILABLE OBJECTIVES

* for Super-resolution PALM imaging. Requires special glass coverslips 0.17 and immersion oil
LASERS
.jpg)
FILTERS FOR OBSERVATION AND ACQUISITION
Observation (oculars):
- One triple bandpass filter (Filter set 25 HE)
Acquisition (Widefield and Super-resolution imaging):
.jpg)
* BP 495-550 / LP 750 is available; ** BP 570/620 / LP 750 is available
.jpg)
BP: Bandpass filter, LP: Longpass filter, LBF: Long Bandpass filter.
DETECTION
.jpg)
SUPER-RESOLUTION TECHNOLOGY
SIM and PALM/STORM are two approaches that aim to break the diffraction limit of conventional light microscopy, and yield up to significant increase in axial and lateral resolution. A non-exhaustive list of publication related to the use of Zeiss LSM880 – Elyra PS.1 for super-resolution imaging can be found here.
Super-resolution SIM imaging uses interference-generated light patterns to create Moiré fringes. Briefly, these fringes resulting from the interference between the sample and a spatially-defined fine diffraction grating placed in an intermediate image plane “downshift” the high frequency image information that were previously not accessible to lower spatial frequencies, which can now be retrieved. By imaging these interferences (moving the grating for 3 or 5 phase positions over 3 angles) and analyzing them, we are able to reconstruct a high-frequency information image*. This technique does not require any specific sample preparation method as well as the use of conventional fluorophores is possible. One can expect a gain of resolution up to approx. two-fold compared to conventional confocal microscopy. However, super-resolution SIM imaging is not optimal for thick and/or densely labelled samples.
For more information on super-resolution SIM imaging, see iBiology SIM and Zeiss SR-SIM.
* For a 2D (resp. 3D) super-resolution SIM image, 3 (resp. 5) positions over 3 angles are necessary, respectively.
Super-resolution PALM/STORM imaging relies on stochastic switching of fluorescent molecules of defined photochemical properties between a bright (i.e. emissive) and a dark (i.e. weakly or non-emissive) state. An optically resolvable subset of fluorophores is activated to a bright state at any given moment, detected and imaged, such that the location of each molecule can be individually determined with high precision in 3D. This subset is subsequently deactivated, and another subset is activated and imaged. Iteration of this process allows the collection of numerous position information of fluorophores for reconstructing a super-resolution image with a lateral (resp. axial) resolution improvement up to approx. 20-50 nm (resp. 50-100 nm*). PALM/STORM requires the use of specific fluorescent probes referred as photoconvertible and photoswitchable. In addition, STORM technique relies as well on specific sample preparation protocols.
For more information on super-resolution PALM and STORM imaging, see iBiology PALM, Zeiss PALM and iBiology STORM.
* Axial resolution improvement is achieved by double phase ramp method. More details, see here.
MISCELLENEAOUS
The facility provides microscopy chambers adapted for live cell imaging (Chamlide).
Best suited for: Fixed and Live samples, Tile Scan, Multi-position acquisition, High-speed increased-resolution confocal imaging, Super-resolution SIM and PALM/dSTORM 3D imaging.
For more information, contact Neurimag facility.
Return
Leica - Spinning disk X1

At a glance, here are the features of this new microscope:
- Top-hat illumination which provides a homogeneous illumination/excitation of your sample over the whole field of view of the camera (designed by Errol).
- Up to 5 laser lines including 405, 488, 532, 561 and 633 nm (a 730 nm laser line should be available soon after).
- Dual camera image splitter (W-View Gemini-2C from Hamamatsu) for high-speed two-colors confocal acquisition. The component will be tuned for fast mEGFP-mCherry imaging. The system will be equipped with two sCMOS (Flash 4.0 v3, Hamamatsu) cameras.
- Environment control (temperature and CO2) to carry out Live sample imaging.
This system will further be upgraded for experiments requiring photomanipulation tools.
Return
Zeiss Axioplan 2

Upright epifluorescence microscope equipped with a CoolSnap camera (Roper Scientific) driven by MetaView (Molecular Devices)
KEY FEATURES
Quick checking of fluorescent staining of your fixed samples with 6 emission filters
Image acquisition using MwetaVue, a user-friendly interface software
AVAILABLE OBJECTIVES
.jpg)
LIGHT SOURCE
HBO (fluorescent, 100W) and HAL (transmitted light, 100W) lamps
FILTERS
In addition to filters for CFP and YFP:
.jpg)
DETECTION
Monochromatic camera CoolSnap ES from Roper Scientific
Return
ZEISS WIDEFIELD SCANNER

Inverted widefield microscope equipped with two cameras (monochromatic and color), a motorized XY-stage, a COLIBRI LED-based illumination (7 lines including a 735/40nm one) for independent control of intensity illumination and sample holders suitable for slide/plate scanning.
KEY FEATURES
User-friendly and complete software for immediate ease of use.
Motorized XY-stage for Tile scan imaging of plates/slides.
Two cameras: Hamamatsu ORCA-Flash4LT and Zeiss Axiocam 305
A 7 solid-state LED lamps (385, 430, 475, 555/590, 630 and 735 nm) and 5 emission bandpass filters (425/30, 514/30, 592/25, 681/45, 788/38)
A module for automated sample detection (AI Sample Finder) for a quick tile scan region definition.
A wide range of objective magnification (dry and oil-immersion ones): from 2.5x to 63x
ADVANCED CAPABILITIES
The Zeiss widefield microscope is equipped for fast tile-scan imaging using independent control of light illumination intensity. The feature AI SAMPLE FINDER is implemented on the system to help you detect and define the contour of your biological sample, ready for tile scan imaging.
Return
Bruker VUTARA 352
Bruker VUTARA 352 microscope is an inverted wide field microscope equipped for 3D dSTORM, PALM, PAINT or Single Particle Tracking with six hight power laser lines (405, 488, 561, 640, 750), and two camera (sCMOS camera (4 MP, 6.5 µm x 6.5 µm pixel size for super-resolution imaging); CCD camera (1392 x 1040 for widefield imaging) allowing multiple color super-resolution microscopy on thick samples (up to 8 microns). It is coupled to a swept-field microscope OPTERRA equipped with an Hamamatsu EMCCD camera, allowing correlative microscopy with tissue tile imaging, or live sample imaging.
KEY FEATURES
Improved user interface for 3D STORM imaging: the SRX software allow you to directly visualize in live, your single molecule reconstruction picture. It can be correlated with widefield picture, tile picture and swept-field pictures. It can aquire sample in 3D (via high-precision piezo Z stage (100 µm option), and along the time (20 µm x 10 µm x 1µm volume at up to 3,000 fps for 3D particle tracking).

AVAILABLE OBJECTIVES
Olympus Water: 60x/1.2;
Olympus Silicone: 60x/1.3;
LASERS
Laser Options 100 mW: 405 nm;
1000 mW: 488 nm, 561 nm, 640 nm, 750 nm,
ENVIRONMENT CONTROL
Temperature control device is available to carry out Live sample imaging.
Return
Workstations
 |
Configuration:
Windows 7 Professional 64bits, Intel Core i7-2700k 3.50 GHz
AMD Radeon R9 380, 32 GB RAM
2 x 128 GB SSD (boot, RAID 0) and two 1.5 TB SATA rpm
Available software:
Volocity, Neurolucida, Icy, FiJi, Pack Office
|
 |
Configuration:
Windows 7 Ultimate 64bits, 2x Intel Xeon E5-2643 Quad Core 3.30 GHz
AMD FirePro V5900 2 GB graphic card, 8 x 8 GB RAM
256 GB SSD (boot) and two 2 TB SATA 7200 rpm (configured as 4 TB RAID 10 hard drive)
Available software:
Icy, Fiji, Zen Blue for online processing of Airyscan images (Zeiss LSM 880 – Elyra PS.1) |
Return
Additional services
|
Microscopy chambers

|
Chamber diameter is 55 mm
Designed for 18 mm round coverslip
1 main body, 1 bottom plate, 1 silicone ring, 1 silicone tubing for perfusion (Ø 2 mm) |
 |
Chamber diameter is 35 mm
Designed for 18 mm round coverslip
1 main body, 1 bottom plate, 1 glass cover, 1 silicone ring |
Support for Image analysis and data processing
Staff can provide support for image processing and data analysis.
Contact the staff for more information.
Sample preparation room
A room for sample preparation can be found in the facility. It comprises a sterile hood, cell culture incubator, lab consumables and disposable lab supplies. Contact the staff for more information.
Return
NeurImag's primary culture service is a unique biological sample preparation service in Ile de France. We provide cultures of neurons, astrocytes and microglia maintained to the point of use (up to several weeks or monthes).
Cells can be recovered at the end of the preparation to be plated in your laboratories or maintained by our service for projects in partnership with NeurImag's Imaging services.
Services are open to internal (Institute of Psychiatry and Neuroscience of Paris) but also to external users (academics or private).
Requests for technical services can be made after a scientific meeting with members of the NeurImag team and via the reservation system on the website.
The primary culture service takes care of the quality of the cells until delivery.
All services of the primary culture (cultures of neurons, transfection and culture of glial cells) are carried out exclusively in laboratories of safety 2 of the IPNP.
All the services provided are invoiced quarterly at the same time as the rest of the facility (imaging and analysis). Prices are available HERE.
For more information, feel free to contact the members of the NeurImag facility: neurinag.ipnp@inserm.fr.
Services
We offer several technical services:
• Weekly culture of primary hippocampal neurons, cortical and glial cells (Astrocytes or microglia)
Requests for primary cells must be made before Friday 10 a.m. to get cells on the following Tuesday. User should take care of the cells upon delivery and take into account the coating of the media whare they plan to seed the cells. The conditioned medium to maintain the cells can be provided by the service on request.
For collaborative projects integrating NeurImag imaging services, cells can be seeded and maintained until they are used by the primary culture service. The supports suitable for the NeurImag platform microscopes (18mm Menzel type 1.5 glass coverslips or 35mm glass petridish) are also offered by the service.
Transfection
Transfections can be performed with lipofectamine 2000 on cell line or cultured neurons of various stages (DIV7 to DIV 14).
Other techniques can be proposed on request, please contact the NeurImag team for further discussion neurinag.ipnp@inserm.fr.
• Fixation and Immuno-staining
The service supplies consumables such as fixing solutions (PFA 4%) and blocking solutions. The antibodies are to be provided by the teams.
These services are provided as part of a collaborative project. Please contact the NeurImag platform neurinag.ipnp@inserm.fr for any request.

Rules and Procedures in NeurImag
Introduction
General rules for access to NeurImag
Available Systems
Training Procedure
Booking Procedure
Data Backup
Rules for Equipment use
Health and safety
Data safety
Reference in literature
1. Introduction
Welcome to the Imaging Facility of the Institute of Psychiatry and Neurosciences of Paris (IPNP) NeurImag.
NeurImag is one of the four core facilities of the IPNP, located in the 14th district of Paris. It operates in direct collaboration with INSERM, the University of Paris Descartes and Sainte Anne hospital.
NeurImag users, once authorized and trained by our team will be free to use the various systems.
Access to the facility implies acceptance of rules and procedures set by the facility and approved by the executive committee of the IPNP.
2. General rules for access to NeurImag
2.1. Location
NeurImag is located on the fourth floor of the IPNP, 102-108 rued e la santé, 75014, Paris.
2.2. Access
→ Metro line 6 : Stop Glacière
→ Metro line 4 : Stop Alésia
→ Tramway line 3 : Stop Cité Universitaire
→ RER B : Stop Cité Universitaire
→ Bus n°21 : Stop Glacière Tolbiac
→ Bus n°62 : Stop René Coty or Stop Glacière Tolbiac
→ Bus n°88 : Stop Alésia - René Coty or Stop Glacière Tolbiac
→ Bus n°216 : Stop Alésia - René Coty or Stop Glacière Tolbiac
→ Bus Orly-bus : Stop Alésia - René Coty or Stop Glacière Tolbiac
2.3. Opening access
For the IPNP staff, opening access is set by IPNP’s access rules: Monday to Friday from 8am to 8pm.
If access is needed outside the usual schedule, including weekends and public holidays, users are requested to apply for a special working time and they must notify their presence in the book dedicated for exceeding hours, available at the reception desk.
Access for external user is authorized from 9am to 5.30 pm, Monday to Friday.
3. Available Systems
The following systems are available for use:
→ Data analysis station 1
→ Data analysis station 2
→ Data analysis station 3
→ Zeiss Axioplan 2 Fluorescence Microscope
→ Zeiss Scanner Fluorescence Microscope
→ Spinning disk Microscope
→ Leica TCS SP5 Microscope
→ Leica TCS SP8 Microscope
→ Zeiss LSM 880 Microscope
→ BRUKER STORM Vutara Microscope
Technical parameters are available on IPNP’s Website
3.1. Billing
Details for billing are available on IPNP’s Website.
4. Training Procedure
4.1. General matters
User’s rules and regulation will have to be signed by the user and his/her team leader prior to any training. In case of material damage, the repairing cost will be charged to the belonging team
To ensure the proper use of the systems, it is recommended to proceed with the training once the research project is clearly defined.
Training will be charged as one hour of use of the corresponding system.
Users are welcome to contact our team to ensure the compatibility of their experiments with the systems of the facility.
4.2. Training enquiry
To apply for training:
Access the training application form: link
Once the form has been filled and submitted to our team, your application will be processed as soon as possible. You will then receive a confirmation email indicating the date and time slot for training.
The facility is open to external users. They must first apply for cooperation.
If you experience any problems related to the request for training, if you need more information, contact our team by email at neurimag.ipnp@inserm.fr
5. Booking Procedure
5.1. General matters
Using the system is open and free pending that NeurImag’s team certifies the ability of users to use the systems.
User can cancel any booked slot up to 24 hours before the beginning of it. Within this period, the user will have to justify it by filling the dedicated form available on IPNP’s website
Booking slots are maintained until 30 minutes after the start of the slots. Beyond this period, if the user is not currently using the system, the reservation is canceled and the time is made available to other users. The slots will be charge anyway unless NeurImag’s team is able to find a substitute.
In case of non-compliance with the booking rules, the imaging facility team has the right to cancel the booking without notice or warning. Thus, users are asked not to reserve systems carelessly. We also ask, if possible, not to book slots larger than four hours per day
5.2. Booking enquiry
Users are asked to make an online booking via IPNP’s Intranet website
Intranet access is reserved for members of the IPNP, users who are unable to connect to it should contact our team at mailto:neurimag.ipnp@inserm.fr.
6. Data Backup
Systems of the imaging facility will in time be connected to the internal network the IPNP. Data backup should therefore be done via this network.
In order to facilitate data storage, for each use, users are asked to back up their experiments in a folder "team" and a subfolder "First name LASTNAME". The file for the experiment will be as 'YYYY/MM/DD + descriptive information. "
To avoid exceeding storage on the hard drives, users are asked to export their data and store it on their own computer. NEURIMAG staff have the right, if necessary, to remove any experiment data stored on hard drives without notice or warning.
Regular data backups will be conducted by the team after warning users and allowing them sufficient time to save the data onto an external storage device.
NeurImag’s system are backed up on a daily basis and NeurImag certify the accessibility of any data within a period of one month.
7. Rules for Equipment use
7.1. Hardware
NeurImag’s staff is responsible for maintaining systems in an optimum state. To achieve this goal, they can at any moment reformat part or all of the hard drives. Such emergency interventions will occur without notice or warning.
Users must use the user sessions of the various computers. They are not allowed to install, uninstall or make updates to software without express authorization of NeurImag’s staff.
Users are not allowed to read or use files or data that do not belong to them.
7.2. Rooms and Systems
The temperature in the rooms containing a microscope is set at 22°C with air conditioning. This temperature is essential to ensure optimal functioning. Users are not allowed to modify it.
Metrology tests are regularly done by NEURIMAG staff in order to ensure users that the equipment is in a good working state and that data obtained are scientifically relevant.
The good performance of the workstation is the responsibility of the user that reserved the time slot. Misuse of the workstation by a user can result in a temporary or permanent loss of NeurImag access.
7.3. Supllies
Some supplies are available to users, namely:
→ Optical paper
→ Immersion oil
→ Paper tissue
→ Lens cleaning solution
→ Latex gloves
→ Bins for contaminated material end samples
→ Demonstration slides (on request)
If any of those supplies is missing, please notify to a NeurImag staff member.
8. Health and safety
8.1. Hardware
In order to guaranty hardware integrity, users are asked not to plug any USB memory stick onto computers.
NeurImag cannot be held responsible for the transmission of computer viruses and other harmful elements to the proper functioning of the user’s hardware.
8.2. Biological material
For safety reasons, we ask users not to bring any infected live cell or toxic/pathogenic reagent onto the Facility. Touching NeurImag’s system with gloves is prohibited since the sample pathogenicity is supposed to be at level 1. It remains possible to bring infected sample if it has been fixed in order to avoid any system contamination. User using human sample, potentially containing prion (even if it is fixed) have to mentioned it to NeurImag in order to evaluate the imaging feasibility. Please contact us is you have any doubt.
8.2. Rooms and systems
Users are asked to respect the cleanliness of the system and keep it in a good working state. Use these simple rules of common sense (not exhaustive):
→ Clean up Oil or Immersion objectives after use.
→ Dispose of contaminated product in the yellow bins provided for this purpose that are available in all NeurImag’s rooms
→ Make sure that media samples (slides, Petri dishes…) do not leak.
→ Do not use potentially contaminated gloves to touch computers and microscopes.
→ Contact NeurImag or CPN staff for health and safety if any problem occurs.
For obvious health and safety reasons, sensitive equipment (slide, Petri dish, culture dish ...) left on a workstation will be immediately discarded without warning or notice by the staff.
9. Data confidentiality
Images and data collected on NeurImag’s systems belong to the team who made the experiment and totally obey to the regulations and practices relating to intellectual property.
Members of the board are not allowed to communicate on research topics of users without the agreement of the teams.
If confidentiality is required, we recommend that users do not store their data over the network or on NeurImag’s computers. In this case we recommend storing data on CD.
10. Reference in literature
10.1. General matters
Mentioning NeurImag or its agents in literature can highlight the merits and usefulness of their presence in the scientific community. Acknowledging NeurImag also facilitates applications for funding to invest in new equipment and new resources.
10.2. NeurImag citation
NeurImag’s name will be cited in any publication including data obtained through its systems. This citation can appear for example in the materials and methods or in the acknowledgments: « We acknowledge NeurImag facility of the Institute of Psychiatry and Neuroscience of Paris where imaging experiments were carried out»
10.3. Team citation
In case of involvement in a collaborative project, NeurImag’s agents involved will be included in the authors of any publication resulting from this work.
Outside the context of collaboration, users are not obliged to include them in the list of authors. However, if the authors believe that the work of a member of the board was critical to obtain the published results, the user can decide whether to include the person in the author list.
The NeurImag platform is part of the UP3 Consortium (University of Paris Photonic Platform)
Missions
The mission of the University of Paris UP3 platform consortium is to offer its expertise, train and provide researchers with the equipment and expertise necessary to carry out fluorescence microscopy imaging projects. It is fully open and accessible not only to teams at the University of Paris sites, but also to external users, whether they are from academic laboratories or private companies. The purpose of this consortium is to guide users to the most suitable sites according to their scientific question and to rationalize the choices when purchasing new equipment, in order to optimize the services available.

Members :
Up3 is made of 7 facilities belonging to the Health faculty and the Sciences faculty of Paris University
Cochin Site :
Cochin photonic microscopy facility - IMAG’IC
(INSERM U1016, CNRS UMR8104, Paris University)
Jacques Monod Institute Site :
Institut Jacques Monod cellular imaging facility - ImagoSeine
(CNRS, Paris University)
Institut of Psychiatry and Neuroscience of Paris (IPNP) Site:
Institut de Psychiatrie et Neurosciences de Paris photonic microscopy facility - NeurImag
(IPNP, INSERM1266, Paris University)
Saints Pères Site:
Centre Universitaire des Saint Pères (CUSP) microscopy facility
(Saints-Pères Paris Institute for the Neurosciences, SPPIN – CNRS UMR 8003, Paris University)
SFR Necker Site:
SFR Necker cellular imaging facility
(INSERM US24, CNRS UMS 3633, Paris University)
Research center for Inflammation Site:
IMA'CRI photonic microscopy facility -
(INSERM UMR 1149, Paris University)
Saint Louis Site:
IRSL technological facility
(Paris University)























.jpg)
.jpg)
.jpg)

.jpg)
.jpg)
.jpg)


.jpg)
.jpg)
.jpg)
.jpg)


.jpg)
.jpg)



















![Light Microscopy _ Metrology tutorial for PSF characterization [720p].mp4](/prod/file/insermcpn/thumbnails/facilities_video10.jpg)